Plants that fight back: scientists engineer disease-resistant crops
A modification to plant proteins helps them recognize and attack pathogens such as viruses.
Crop diseases are a huge drain on our food supply. The soybean mosaic disease, for example, is a highly destructive infection that leads to billions of dollars in losses on soybean yields every year around the world.
For decades, farmers have tried to battle crop diseases with pesticides, but these agrochemicals often can harm non-target animals and microorganisms, disrupt ecosystems, and contaminate soil and water sources. Worst still, many pathogens or pests adapt and develop resistance to chemicals, reducing their effectiveness.
Plant biologists at Tsinghua University are taking a different approach to bolster plant defenses against disease. In a recent study published in Nature1, researchers led by Yule Liu, have enhanced the immune systems of plants by reengineering proteins to act as more vigilant sentinels.
These modified proteins can improve the plant’s capacity to recognize invading intruders and trigger the its immune defenses. “Our approach targets the root of disease susceptibility: the plant’s ability to detect pathogens,” says Liu.
Vigilant sentinels
Central to this breakthrough is an ‘alarm protein’ found within plant cells, known as ‘nucleotide-binding domain and leucine-rich-repeat immune receptors’, or NLRs.
NLRs can detect specific pathogen-or pest-derived molecules introduced into plant cells by bacteria, fungi, viruses or insects. These receptors then become activated and initiate a cascade of immune responses against the foreign pathogens, such as killing off the infected cells to prevent the spread of infection.
“It is like a guard sounding an alarm during an invasion,” Liu explains.
However, some pathogens have evolved to evade detection by avoiding the production of the molecules that would normally activate NLRs. Moreover, NLRs cannot stay activated all the time, as that would cause excessive damage to healthy plant tissue. This mirrors the human immune system, where hyperactivity can lead to autoimmune disorders, such as Crohn’s disease.
In 2017, while examining the functional mechanism of NLRs, Liu’s team reported that an activated NLR will stop working if one of its terminal regions was blocked2. “Reports from other labs suggested this was a common issue,” recalls Liu.
Rather than viewing it as a flaw, the team saw potential. They hypothesized that if they could design a custom-built blocker that only a pathogen can remove, and attach it to an active NLR, they could effectively create a molecular trap.
In its blocked state, the NLR would remain dormant. But upon invasion, the pathogen could remove the blocker, automatically unleashing the NLR and triggering the plant’s immune defenses. The team dubbed this innovation ‘controllable autoactive NLRs’, or ‘controllable aNLR’ for short.
Sustaining immunity
To achieve broad-spectrum resistance against multiple pathogens, the blocker on aNLRs would be attached with short peptide sequences that can be cut by enzymes produced by a wide range of pathogens.
The researchers first focused on potyviruses, a large viral family that accounts for about 30% of all known plant viruses, including the soybean mosaic virus. They identified a conserved protease called NIa — a type of enzyme that plays a crucial role in breaking down polyproteins into smaller, active mature proteins — which is essential to the infection process of all potyvirus species. Moreover, the NIa cleavage sites from more than 100 potyviruses are highly conserved. The researchers then engineered a blocker peptide sequence containing the conserved cleavage site, and attached it to the beginning (N-terminus) of the aNLR.
Through this strategy, the tools that viruses use for infecting cells are repurposed as ‘molecular scissors’ that activate the aNLRs. “This in turn sounds the alarm for the plant’s immune system to launch a defense,” explains Liu.
The resulting plant immunity is expected to be long-lasting and difficult for viruses to develop resistance to, because the enzymes involved are critical for the pathogens to infect the plant and unlikely to be lost through mutation.
To test this approach, the researchers used a common model plant, Nicotiana benthamiana. After designing the aNLRs, they introduced them into the plants using standard genetic engineering techniques, enabling the plants to produce the modified receptors themselves.
In a trial spanning five different viruses — including the potato virus Y (PVY), turnip mosaic virus, pepper mottle virus, chili veinal mottle virus, and the plum pox virus — between 70% and 100% of plants that carried the engineered aNLRs did not get systemic infections, compared to zero resistance in the control group. The engineered plants even maintained their resistance when the researchers tried to infect them with all five viruses simultaneously.
“We were initially surprised by the robustness of resistance, that one engineered aNLR could protect against multiple potyviruses,” Liu says.
However, the engineered plants did not show similar resistance against the tobacco etch virus (TEV), succumbing to systemic infections. The team then created another aNLR with two cleavage sites, each responsive to different viral enzymes. They managed to raise the complete resistance rate against TEV to 55% while maintaining 100% complete resistance against PVY. Further research would be needed to improve the rate, explains Liu.
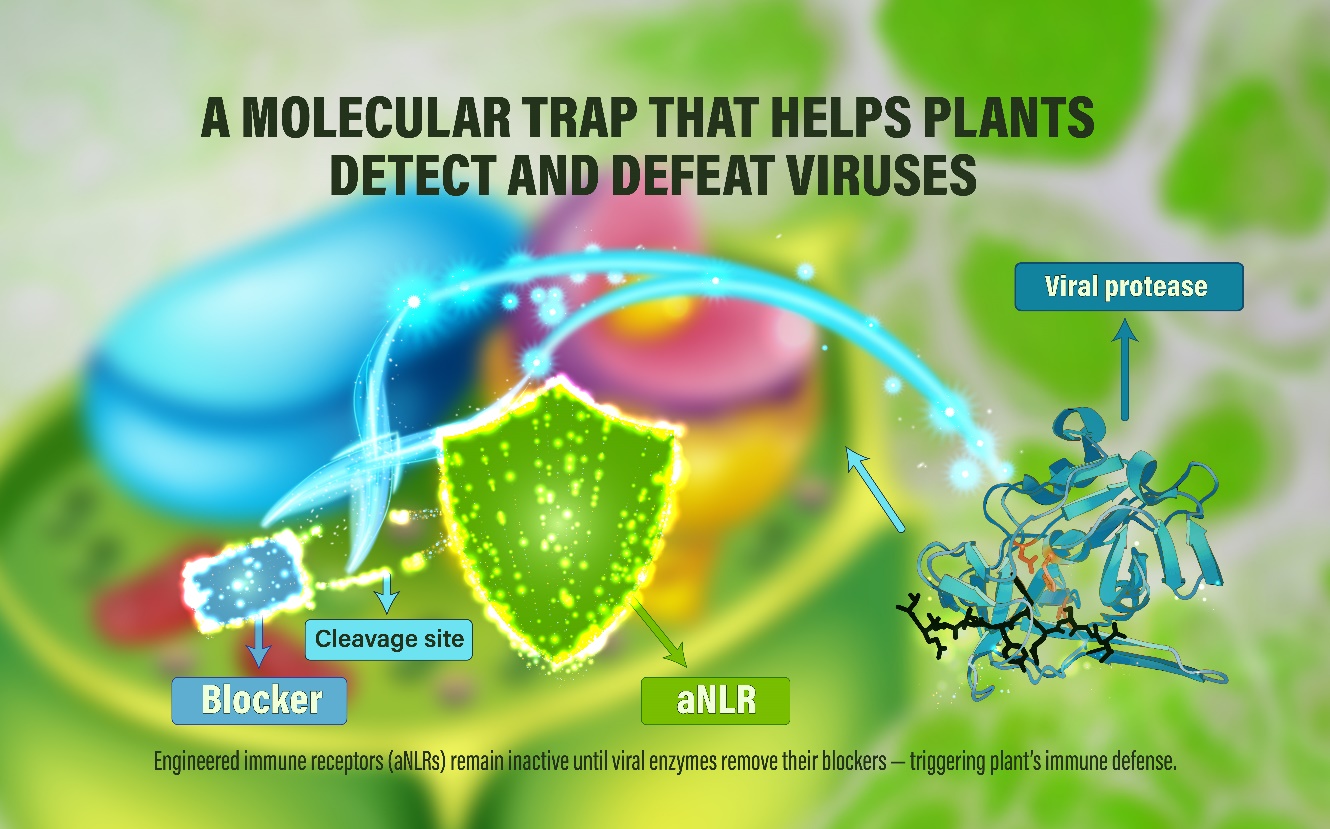
Caption: Engineered immune receptors (aNLRs) stay silent until viral enzymes act like ‘molecular scissors’ to remove their blockers, triggering a powerful immune response against viruses.
Credit: Tsinghua University
Thriving crops
To evaluate the effectiveness of their strategy in a real-world crop, the researchers engineered soybean plants and exposed them to the soybean mosaic virus. Compared to unmodified plants — which all succumbed to the virus — the soybean plants carrying aNLRs were able to withstand the virus and continued growing normally, showing no viral symptoms and no detectable viral RNA.
So far, the researchers have not observed abnormalities or other side effects in the growth of genetically modified soybean plants, but field trials are still needed to assess long-term impacts, Liu says. The team also aims to employ CRISPR genetic editing techniques to modify native NLR genes directly in crops without introducing foreign genetic material. This approach would help allay concerns associated with transgenic crops, Liu says.
Beyond viral targets, the researchers are working to design aNLRs with blockers that bacteria, oomycetes, fungi, nematodes and piercing-sucking pests could remove. A major focus of this effort is to identify enzymes that these microbes commonly produce during infection. “By engineering immune receptors, we equip crops with ‘built-in armor’ that reduces yield loss and promotes ecological sustainability,” Liu says.
A profile photo of Prof. Liu
Caption: Yule Liu Xu is a professor at the School of Life Sciences at Tsinghua University. He is widely recognized for his research in plant molecular biology and immunity, particularly in understanding how plants defend themselves against viruses and other pathogens.
Credit: Tsinghua University
Liu is also leading research to advance understanding of innate plant defense mechanisms beyond NLR-mediated immunity. One area of interest is the airborne defense mechanism, where plants emit volatile organic compounds in response to aphid attack. These airborne cues serve as early warnings to neighboring plants, prompting them to produce other compounds to counter the aphids and the viruses that they transmit3.
Ultimately, Liu hopes that his team’s research would create crops that can withstand diseases on their own. “Our goal is to see these disease-resistant crops thrive in fields, protecting harvests and securing food for future generations,” Liu says.
References:
1.Wang, J., Chen, T., Zhang, Z. et al. Remodelling autoactive NLRs for broad-spectrum immunity in plants. Nature (2025).
2.Chen, T. et al. Antiviral Resistance Protein Tm-22 Functions on the Plasma Membrane. Plant Physiol. 173, 2399–2410 (2017)
3.Gong, Q., Wang, Y., He, L. et al. Molecular basis of methyl-salicylate-mediated plant airborne defence. Nature 622, 139–148 (2023).
