Across evolution, interactions between the sexes have been essential for reproduction and survival. Yet one longstanding mystery remains: why does a female’s interest in sociosexual interactions rise and fall with her reproductive cycle? Female interest in mating typically peaks around ovulation, driven by hormonal changes. While the hormonal influence on mating behavior has been well documented, most studies have focused on deep brain regions like the hypothalamus. Less is known about how hormone signals are interpreted by the brain’s higher-order areas involved in decision-making and motivation.
In a new study published in Cell on May 20, 2025, Wang et al. uncover a hormone-sensitive circuit in the prefrontal cortex that links internal reproductive state to flexible, sex-specific sociosexual behaviors. Led by Dr. Kun Li at Tsinghua University, in collaboration with Dr. Nathaniel Heintz at Rockefeller University, the team identified a population of Cacna1h-expressing neurons in the medial prefrontal cortex (mPFC) that integrate internal reproductive states with external social cues to adaptively guide social behaviors.
This study highlights a previously unrecognized top-down pathway through which the cortex dynamically adjusts behaviors based on internal physiological state. By tuning sociosexual interest to hormonal conditions, this circuit enables flexible, context-appropriate responses to the social world. These findings offer a new lens into how hormones shape complex social decisions and help explain why disorders of social motivation often differ between the sexes.
A Prefrontal Sensor of the Estrous Cycle
Using single-cell RNA sequencing, circuit tracing, and electrophysiology, the team identified a subtype of deep-layer pyramidal neurons in the mPFC that are especially sensitive to hormonal changes across the female reproductive cycle. These neurons send signals to the anterior hypothalamic nucleus (AHN) and become more excitable during the fertile phase of the cycle, due to increased expression of Cacna1h—a gene that encodes the Cav3.2 calcium channel.
When females interact with males during this fertile window, oxytocin released in the brain inhibits Cacna1h-expressing neurons in the mPFC. But because these neurons express high levels of Cav3.2, this inhibition paradoxically causes them to rebound excitation and fire strongly, amplifying their response to male cues. This in turn activates hypothalamic regions involved in mating behavior. In short, these neurons act as a hormonal sensor—primed to detect sociosexual cues more effectively when the chances of reproduction are highest.
Turning Sociosexual Interest On or Off
To test whether these prefrontal neurons directly shape social behavior, the researchers used chemogenetics to either activate or silence Cacna1h-expressing neurons in the mPFC. When these neurons were silenced in estrous females—normally a period of high sexual receptivity—females showed reduced social interest in males. In contrast, activating the same neurons in non-receptive females made them behave more like they were in estrus: they spent more time approaching males and were less sexually rejective.
Surprisingly, manipulating the same neurons in males produced the opposite effect. In male mice, silencing these neurons increased sexual interest in females, while activating them suppressed it. These opposing effects appear to reflect sex-specific differences in how the neurons respond to social cues: in estrous females, the neurons become excitable and responsive to male presence; in males, lower Cav3.2 channel activity leads to suppression in response to female cues. This difference in how the neurons work in males and females may reflect evolutionary pressures: females benefit from actively choosing mates during fertile periods, while males may need to avoid excessive courtship to conserve energy and reduce competition. These findings show that the same prefrontal circuit can be flexibly shaped by both hormonal state and biological sex.
Reading Hormones States and Social Signals at the Same Time
To understand how these neurons process social interactions in real time, the team used calcium imaging to monitor activity during social behavior. They found that Cacna1h-expressing mPFC neurons encoded two key pieces of information: the sex of the interaction partner and the animal’s own hormonal state. During opposite-sex encounters, these neurons were robustly activated in estrous females but were suppressed in males and in non-estrous females. This bidirectional activity pattern reveals a state-dependent gating mechanism that flexibly regulates social responsiveness based on both external context and internal hormonal state. By combining these signals, the circuit enables animals to adapt their social behavior to match physiological readiness, laying the groundwork for context-dependent social decision-making.
Broader Implications for Understanding Social Motivation and Sexual Health
This study provides the first direct evidence of a hormone-sensitive circuit in the prefrontal cortex that links internal reproductive states to social behaviors toward potential mates. While much prior work has focused on the hypothalamus in regulating sexual behavior, this work highlights a top-down cortical mechanism through which the brain flexibly adapts social decisions to both physiological need and external social context. From an evolutionary perspective, this raises the possibility that the prefrontal cortex played a key role in shifting sexual behavior from instinct-driven responses to more deliberate, cognitively guided decisions—especially in species like humans.
The findings also carry important implications for human health. Dysfunction in this cell type, or in Cav3.2 channel function, may contribute to sex-specific conditions such as low sexual desire in females or increased sexual impulsivity in males.
These conditions often co-occur with mental disorders and differ in prevalence between the sexes. By identifying a neural population with sex- and state-dependent control over social motivation, this work opens new avenues for understanding and potentially treating disorders involving sexual behaviors and hormonal imbalance.
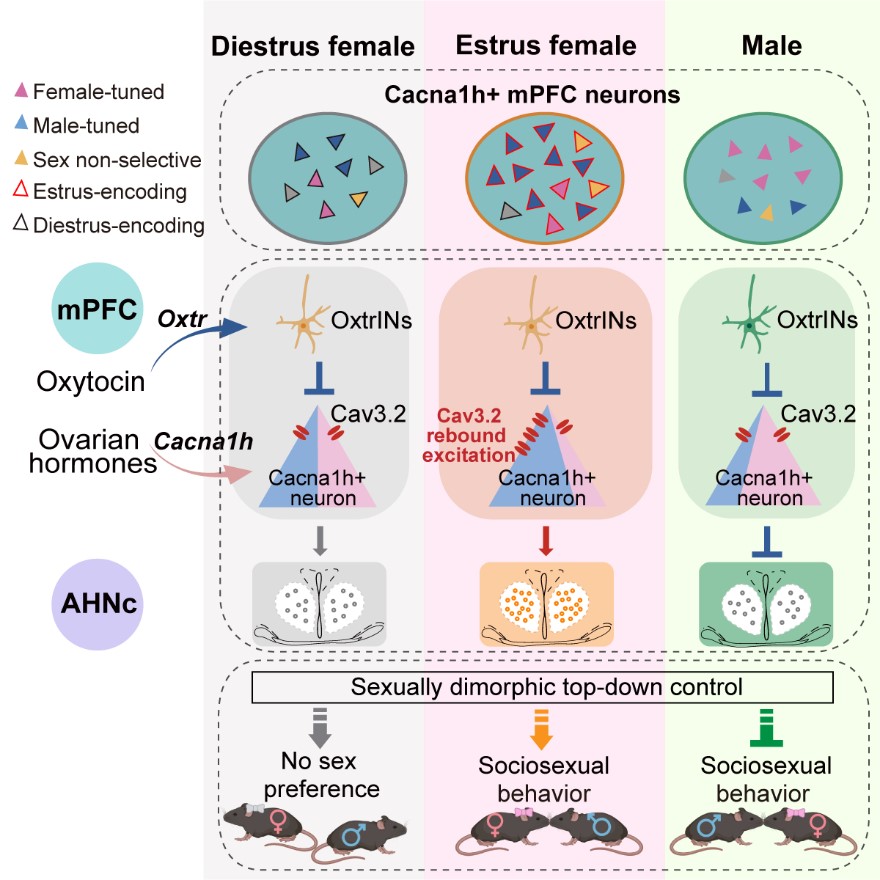
Top-down regulation of sociosexual behavior by Cacna1h+ mPFC neurons integrating social cues and estrous states
