As global temperatures continue to rise, understanding how plants perceive and respond to heat stress has become a critical challenge for ensuring agricultural productivity and food security.
In a groundbreaking study published in Cell Research, a team led by Associate Professor Xiaofeng Fang from School of Life Sciences at Tsinghua University, in collaboration with researchers from Shenzhen Bay Laboratory, has identified a novel plant thermosensor that plays a pivotal role in heat-induced stress granule formation.

Figure 1. The name FUST1 was inspired by the mythological "Fusang Tree" described in the ancient Chinese text Shan Hai Jing, symbolizing FUST1’s intrinsic disordered structure and its central role as a scaffold in assembling stress granules, likened to suns resting on its branches.
The study, titled "A thermosensor FUST1 primes heat-induced stress granule formation via biomolecular condensation in Arabidopsis", unveils FUST1, a previously uncharacterized protein that undergoes temperature-dependent phase separation. Under normal conditions, FUST1 is evenly distributed in the cytoplasm. However, upon heat stress, it rapidly and reversibly forms condensates — a hallmark of stress response.
Biochemical and molecular simulations reveal that FUST1 contains a prion-like domain (PrLD) that acts as a "temperature switch." As temperature increases, this domain undergoes a conformational change from a "locked" to an "open" state, promoting intermolecular interactions and initiating phase separation. This dynamic response enables FUST1 to function as a molecular thermosensor.
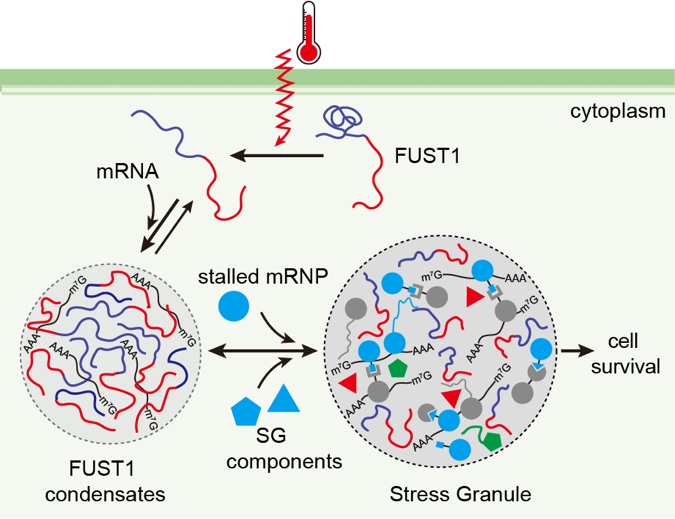
Figure 2. A model illustrating the role of FUST1 in sensing temperature and priming stress granule assembly via its condensation.
Importantly, the research shows that FUST1 condensates form prior to classical stress granules and actively recruit their components. Plants lacking FUST1 exhibit delayed stress granule assembly and reduced heat tolerance, underscoring FUST1's essential role in thermal adaptation. Homologs of FUST1 and its conserved PrLD domain are found across land plants, highlighting its evolutionary significance.
"The study not only deepens our understanding of temperature sensing and stress response in plants but also identifies FUST1 as a promising molecular target for engineering heat-resilient crops," says Professor Xiaofeng Fang.
Link: https://www.nature.com/articles/s41422-025-01125-4
