RNA interference (RNAi), a highly conserved RNA silencing mechanism, plays pivotal roles in gene expression regulation, antiviral defense, and genome protection against transposon activity. Compared to the well-characterized small RNA interference pathways mediated by microRNAs (miRNAs) and exogenous small interfering RNAs (exo-siRNAs), current understanding of the processing, maturation, and functional mechanisms of endogenous small interfering RNAs (endo-siRNAs or esiRNAs), which are longer and possess complex structural features, remains relatively limited.
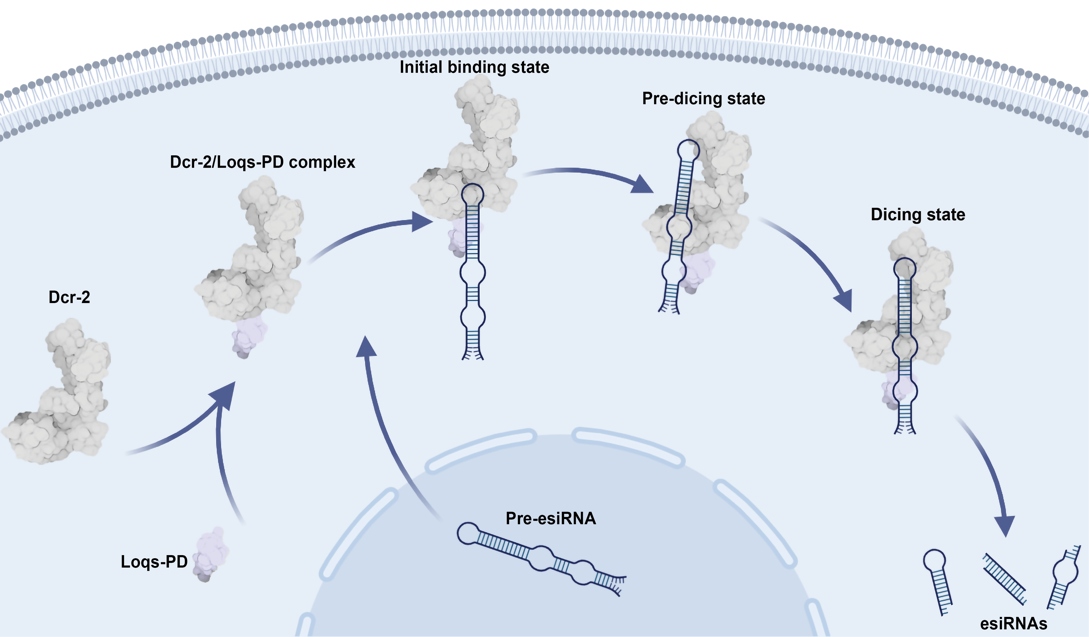
Figure 1. The model of the pre-esiRNA processing by the Dcr-2/Loqs-PD complex
On February 20, 2025, a collaborative study by research teams led by Prof. Hong-Wei Wang and Associate Researcher Dr. Jia Wang from the School of Life Sciences at Tsinghua University, together with Prof. Jinbiao Ma from Fudan University, was published in ‘Nucleic Acids Research’ under the title "Structural basis of endo-siRNA processing by Drosophila Dicer-2 and Loqs-PD." Utilizing cryo-electron microscopy (cryo-EM) single-particle analysis combined with in vitro biochemical assays, this research elucidated the structural mechanism underlying the maturation of esiRNAs through cleavage by Drosophila Dicer-2 (Dcr-2) and revealed the essential role of Loqs-PD in this process (Figure 1). The study focused on two pre-esiRNAs, esi-1 and esi-2, which are the most extensively reported endogenous substrates in Drosophila cells.
Under conditions supplemented with ATP and Mg⟡⁺, the team resolved high-resolution structures of the Dcr-2/Loqs-PD complex interacting with pre-esiRNA in both the pre-dicing state and a higher-resolution dicing state. These structures offered unprecedented insights into the dynamic interactions between Dcr-2 and its RNA substrates. During the transition from the pre-dicing to the dicing state, significant conformational rearrangements were observed in the Helicase and DUF283 domains of Dcr-2. In the pre-dicing state, pre-esiRNA reached the Platform/PAZ domains, but stable interactions were not established until ATP hydrolysis drove further translocation of the RNA substrate. This process involved a 1-nucleotide (nt) shift of the 3ʹ end into the binding pocket of the PAZ domain, ensuring precise positioning for cleavage.
Additional structural analyses of Dcr-2/Loqs-PD/pre-esiRNA complexes in the absence of ATP (representing the initial binding state) and Dcr-2/pre-esiRNA complexes lacking Loqs-PD (representing the loading state) revealed critical functional insights. Without Loqs-PD, pre-esiRNA binding induced substantial domain rearrangements in Dcr-2, displacing the substrate from the RNase III catalytic domains and rendering cleavage incompetent. These findings underscore Loqs-PD's indispensable role in stabilizing substrate engagement within the Helicase domain for effective processing. Complementary biochemical assays demonstrated that Dcr-2/Loqs-PD preferentially initiates cleavage from the structured closed ends rather than the flexible ends of complex pre-esiRNAs.
Acknowledgements
Prof. Hong-Wei Wang, Associate Researcher Dr. Jia Wang, and Prof. Jinbiao Ma served as co-corresponding authors. Co-first authorship was shared by Tsinghua Ph.D. candidate Na Cao, Associate Researcher Dr. Jia Wang, and Dr. Deng Ting from Fudan University.
We acknowledge the Tsinghua University Branch of the China National Center for Protein Sciences (Beijing) and Shuimu BioSciences Ltd. for providing the cryo-EM facility support and the computational facility support. This research was supported by the National Natural Science Foundation of China, National Key Research and Development Program of China, Beijing Advanced Innovation Center for Structural Biology, and Tsinghua-Peking Joint Center for Life Sciences.
Original Article:
https://doi.org/10.1093/nar/gkaf102
