On April 1, 2022, Liang Ge's research group at the School of Life Sciences, Tsinghua University published a research article online in the journal "Cell" titled with “CCT2 is an aggrephagy receptor for clearance of solid protein aggregates”, discovered a novel aggrephagy receptor CCT2 and its important role in the clearance of solid protein aggregates.
Aggrephagy is an important way to clear intracellular toxic protein aggregates and potential therapeutic target for many aggregates-related human diseases such as neurodegenerative disorders. How autophagosomes selectively recognize protein aggregates during aggrephagy has been an important issue, and the mechanism remains to be further studied. Traditional aggrephagy receptors (P62, NBR1, and TAX1BP1, etc.) mediate aggrephagy by binding ubiquitin chains on aggregates and the LC3 on autophagosome membranes. However, because of the nature of these receptors to bind ubiquitin chains, they are also involved in other types of ubiquitin-related selective autophagy. More importantly, these aggrephagy receptors prefer to degrade aggregates with liquidity, but helpless in clearing pathogenic solid aggregates. Therefore, it is important to find new types of autophagy receptors specifically targeting solid aggregates.
In this study, researchers discovered a new type of aggrephagy receptor CCT2 which promotes the autophagic clearance of multiple toxic protein aggregates associated with neurodegenerative diseases. Like traditional aggrephagy receptors, CCT2 can bind both LC3 and protein aggregates. The difference is that CCT2 binds protein aggregates through its apical domain in a ubiquitin-independent manner, which provides the basis for its specificity. Importantly, CCT2 and traditional aggrephagy receptors have great difference in the choice of aggregate state: traditional aggrephagy receptors prefer to degrade aggregates with liquidity, whereas CCT2 prefers to select solid aggregates. Therefore,compared to known receptors, CCT2 is more likely to function in pathological conditions and become a therapeutic target (Figure 1). CCT2 is a subunit of the chaperonin complex that acts as a chaperone to help misfolded proteins correctly fold. Researchers found that CCT2 mediates aggrephagy in a monomeric form because only the CCT2 monomer can expose the VLIR motif for LC3 binding. Interestingly, the presence of protein aggregates hinders the formation of chaperonin complex, thereby releasing more CCT2 monomers to mediate aggrephagy. The conformational change of CCT2 from a complex to a monomer results in its functional switch from a chaperone to an aggrephagy receptor, which both prevents misfolded proteins aggregation in early stage and accelerates later protein aggregates degradation, providing an efficient way to maintain intracellular protein homeostasis (Figure 2).
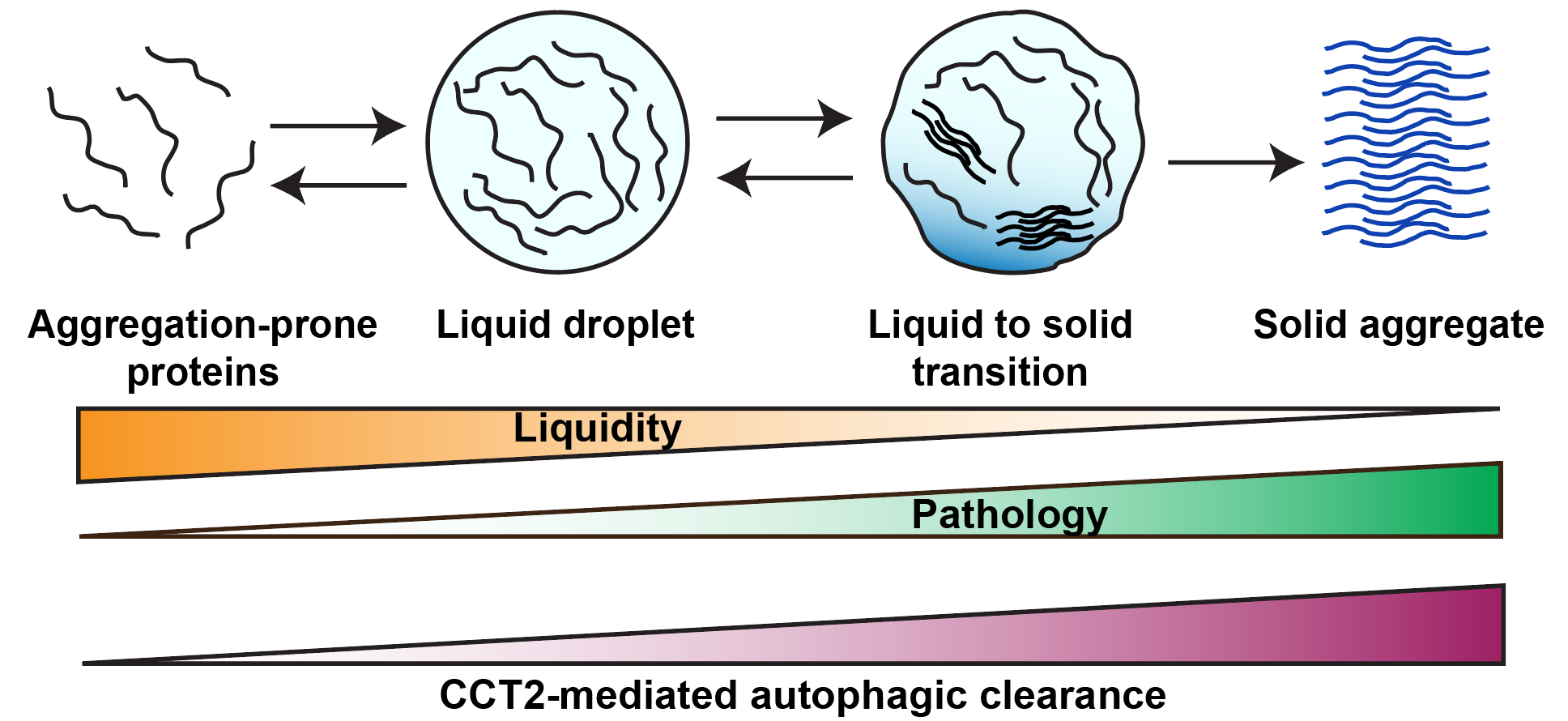
Figure 1. Aggregates phase transition and CCT2-mediated clearance of solid aggregates
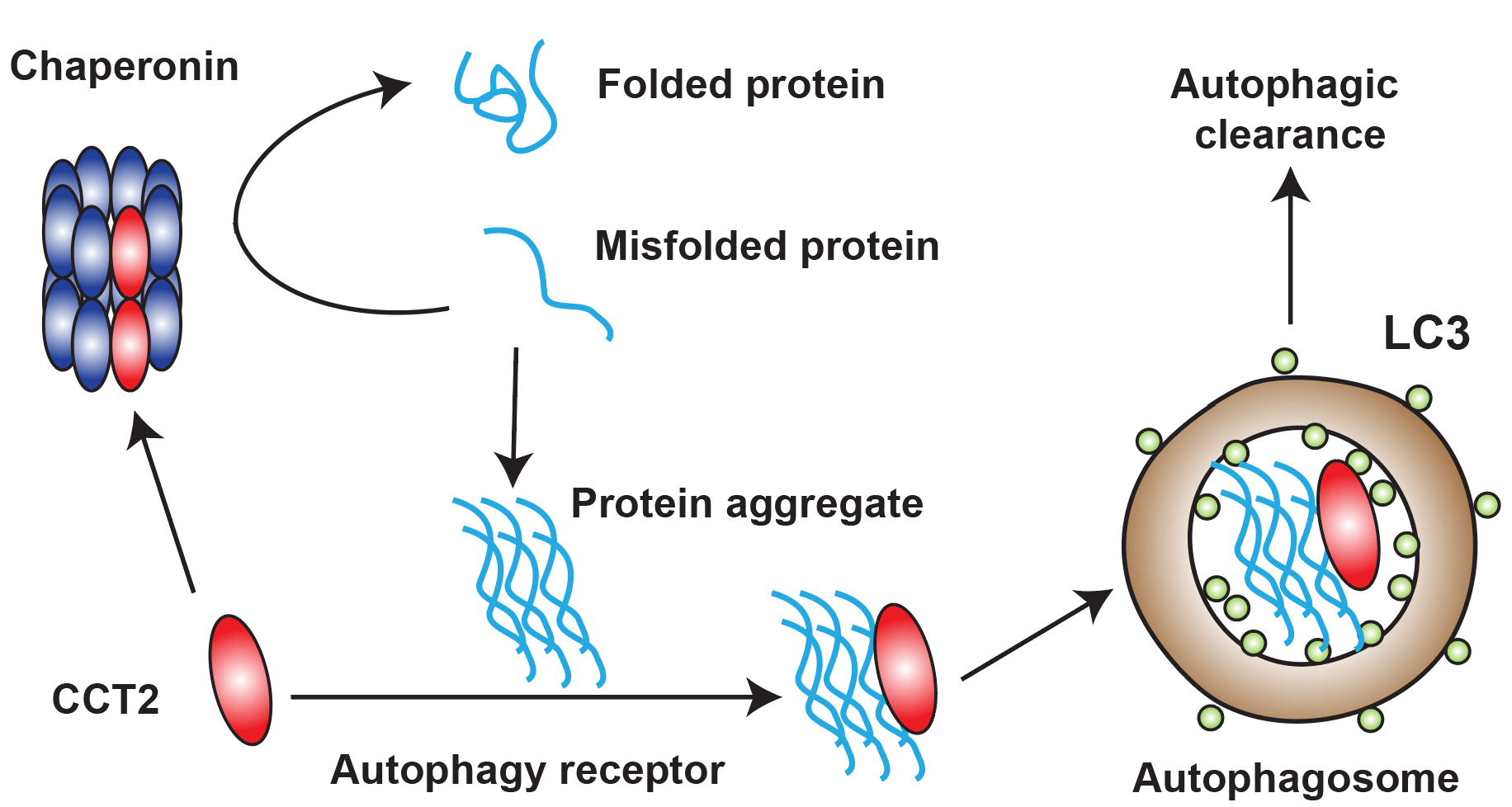
Figure 2. Functional switch of CCT2 from a chaperonin subunit to an autophagy receptor
Dr. Liang Ge (School of Life Sciences, Tsinghua University), Dr. Min Zhang (School of Pharmaceutical Sciences, Tsinghua University), and Dr. Cong Yi (Zhejiang University School of Medicine) are co-corresponding authors and Ph.D. student Xinyu Ma is the first author of this article. Ph.D. student Caijing Lu made important contributions to this research. Ph.D. student Yuting Chen(Zhejiang University School of Medicine), Shulin Li, Ningjia Ma and Tao Xuan participated in the experiments. Dr. Pilong Li, Dr. Haiteng Deng and Dr. Yong-Bin Yan also provided important help to this work.
The work was supported by the State Key Laboratory of Membrane Biology, Ministry of Science and Technology of the People’s Republic of China, National Natural Science Foundation of China, Beijing Natural Science Foundation. Tsinghua Independent Research Program, Tsinghua University COVID-19 Emergency Aid Fund. Zhejiang Provincial Natural Science Foundation of China, Tsinghua-Peking Center for Life Sciences.
Original Article Link:https://www.cell.com/cell/fulltext/S0092-8674(22)00265-3
