Memory forms when a previously neutral stimulus (CS+) becomes competent to predict a biologically potent stimulus (US). But if the CS+ is repeatedly presented without the US after the memory formation, this memory will be suppressed by newly formed extinction memory. Extinction learning-induced suppression of memory has recently attracted major efforts for the development of psychiatric disorders treatment such as post-traumatic stress disorder (PTSD) and drug addiction. An intriguing feature of the extinction of both aversive and appetitive memory is its need of multiple learning trials in leading to the formation of extinction memory; furthermore, even extended learning trials still result in a memory that cannot be sustained long enough to prevent relapse of the extinguished original memory, a feature that is called ‘spontaneous recovery’. This feature has become a major obstacle for translational research. Although extensive efforts have been devoted to the mechanisms underlying the requirement of multiple learning trials by extinction memory and its transient nature, these features remain poorly understood.
A recent report from Yi Zhong’s group demonstrates that the extinction of 24-hour appetitive long-term memory (24-hr apLTM) requires multiple learning trials and shows the spontaneous recovery feature in Drosophila. This provides an opportunity to study the memory extinction in a relatively simple organism with plenty of genetic tools and the best characterised circadian system. In addition, the formation ability of memory extinction has been revealed to oscillate across the day, so we tried to link the unique features of memory extinction to the clock network in Drosophila’s 24-hour appetitive long-term memory. This work results in the discovery of a novel role of clock neurons in gating memory extinction, which is published in the Current Biology on February 5, 2021 (paper link https://www.cell.com/current-biology/fulltext/S0960-9822(21)00041-5).
We report in this paper that inhibiting the activity of clock neurons blocks the formation of extinction memory. Further investigation attributes this role to a subset of cryptochrome-positive dorsal neurons 1 (DN1s) and their downstream SIFamide neurons. The requirement of clock neurons comes from a gating mechanism of extinction for a single extinction learning trial robustly causes typical extinction when coupled with acute activation of DN1s, as marked by the initially enhanced, but eventually diminished memory suppression (Figure 1). Accordingly, we detected specific neural responses to extinction training in a few DN1s via calcium imaging. These data converge in support of the working model elaborated below. Multiple trials of extinction learning first robustly activate DN1 clock neurons. When coupled with this activation, a single trial is sufficient to robustly cause transient extinction of 24-hour apLTM. Conversely, no extinction is permitted if DN1s activation is suppressed. This model implies that only a single trial occurs for the pairing of CS+ with no-US during the multiple extinction learning trials (Figure 2). Considering a single learning trial has been shown to cause short-term memory at a higher rate, this model explains the reason why multiple extinction learning trials fail to cause the formation of long-lasting extinction memory.
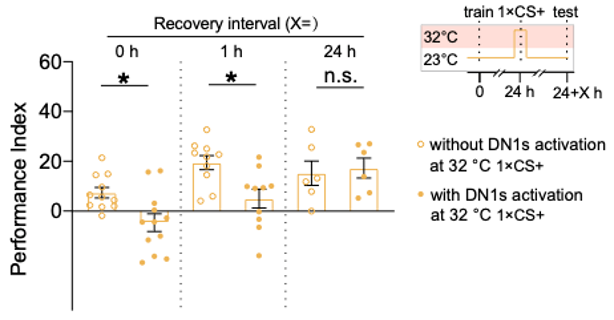
Figure 1. The single-trial extinction learning (1×CS+) is potentiated by the DN1 activation, compared with that without DN1 activation.
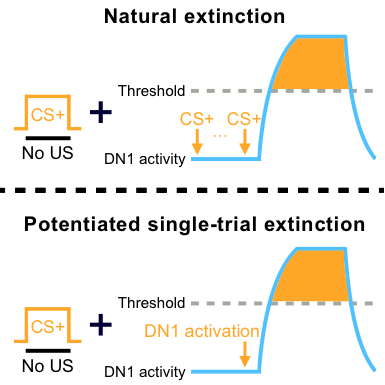
Figure 2. Working model. Multiple trials of extinction learning first robustly activate DN1 clock neurons. When coupled with this activation, a single trial is sufficient to robustly cause transient extinction of 24-hour apLTM.
This study has been published online in the Current Biology on February 5, 2021. Professor Yi Zhong is the corresponding author of this paper. Doctor candidates Yunchuan Zhang and Yinzhong Zhou are the co-first authors. Other doctor candidates Xuchen Zhang and Lingling Wang made important contributions to the paper. This study was funded by the Tsinghua-Peking Center for Life Sciences, the National Science Foundation of China (91332207 and 91632301, to Y.Z.), and the Beijing Municipal Science and Technology Commission (Z161100002616010, to Y.Z.).
