Over evolution, members of the biology kingdom have acquired protein secretion as a fundamental mechanism for intercellular communication. In eukaryotes, majority of the secretory proteins contain a signal peptide which allows for the signal recognition particle (SRP) binding followed by translocation of the cargo into the endoplasmic reticulum (ER) via the translocon SEC61. The cargoes are then exported through ER-Golgi trafficking mediated by COPII and COPI vesicles. The overall process is termed as conventional secretion. The core concepts of this pathway have been included in the textbook of cell biology.
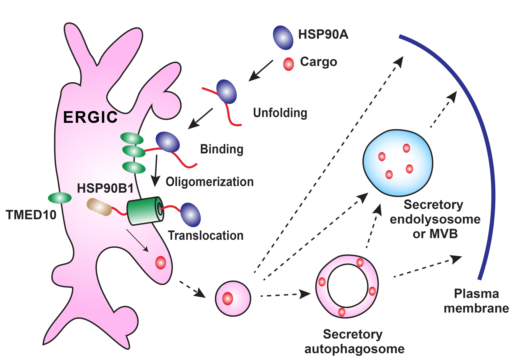
Figure 1. TMED10-channelled UPS
During secretion, a UPS cargo binds to the cytosolic HSP90A which may facilitate the unfolding of the cargo. The unfolded cargo then binds to TMED10 on the ERGIC which induces TMED10 oligomerization to form a protein channel. With the help of HSP90B1, the TMED10 channel translocates the cargo into the lumen of the ERGIC and enters into vesicle trafficking route. The ERGIC may generate the UPS cargo-containing vesicles which either fuse with the plasma membrane or become a secretory autophagosome or endolysosome for secretion.
Importantly recent evidence indicates that many cytosolic proteins lacking a signal peptide are released through unconventional protein secretion (UPS) that bypasses the ER-Golgi trafficking itinerary. Expanding evidence indicates that cargoes which undergo UPS are involved in diverse biological processes (including inflammation, development, virus infection, and lipid metabolism) and human diseases (such as neurodegeneration and cancer). A lot of UPS cargoes rely on vesicular trafficking for secretion, in which the cargoes must enter into a vesicle carrier. Because the cargoes lack a signal peptide, a mystery in field has been how the UPS cargoes enter into the vesicle carrier.
Researchers from the Ge lab in the School of Life Sciences at Tsinghua University found a protein translocation pathway regulating UPS cargo entry into the vesicle carrier. A transmembrane protein TMED10 was identified as a regulatory factor for the secretion of multiple cargoes, including the mature form of interleukin 1(IL1) family members and a set of other UPS cargoes (HSPB5, Galectin-1/3 and Annexin A1). TMED10 directly facilitates the translocation of leaderless cargos into the ER-Golgi intermediate compartment (ERGIC) via its C-terminal tail binding to a certain motif, likely to be a UPS signal peptide, in the cargoes, the process of which is dependent on protein unfolding and enhanced by chaperones HSP90A/HSP90AB1 and HSP90B1/GRP94. Furthermore, the cargo triggers oligomerization of TMED10, which likely then forms a protein channel on the membrane to translocate the UPS cargoes into the vesicle (Figure 1). The pathway identified was termed TMED10-channelled UPS (THU). It is likely that THU acts together with well-known SRP-SEC61 pathway to govern two types of protein secretion e.g. unconventional and conventional secretion, respectively.
The study was completed by the assistant investigator Dr. Min Zhang and Ph.D. candidate Lei Liu. It was published online in Cell journal on April 8th, 2020, entitled “A translocation pathway for vesicle-mediated unconventional protein secretion”. The National Science Foundation of China and the Ministry of Science and Technology of China provided financial support for the work.
https://doi.org/10.1016/j.cell.2020.03.031
