BD is a severe neuropsychiatric disorder characterized by intermittent episodes of mania and depression. However, the origin of BD has remained enigmatic, mainly because genetic animal models based on the currently identified susceptible genes have often failed to show spontaneous mood swings, which are the core feature of BD.
In a paper published online in the Proceedings of the National Academy of Sciences on February 10, 2020, Jun Yao of Tsinghua University and coauthors in China, Canada and the United States described a new strategy for investigating the origins of bipolar disorder (BD) as the result of deficits in multiple genes. In addition to helping to explain why the pathogenesis of BD has been elusive, the work identifies a gene (synaptotogmin-7) common to both bipolar disorder and a metabolic syndrome in mice, potentially pointing to new therapeutic targets for BD.
Interestingly, although pedigree analysis has suggested a very strong heritability of BD symptoms, it has been unclear how a wide variety of genetic deficiencies in the parent can be stably inherited by the majority of offspring. Previously, using induced pluripotent stem cell (iPSC) technology, the authors carried out RNA-seq (which measures gene activity in terms of protein output) and functional analyses in neurons derived from patients with sporadic BD. They found that these neurons showed highly consistent gene activity profiles and shared many cellular qualities. Hence, the authors proposed that regardless of the diverse genetic deficiencies carried by these individuals, they probably share identical molecular deficits that lead to the mood symptoms characteristic of BD.
Given the strong inheritability and characteristic behavioral symptoms of BD, the authors considered that in a subgroup of BD patients, some of the involved molecular pathways might play a central role in the pathogenesis, whereas others are auxiliary or secondary. Therefore, they propose a tree model to explain their hypothesis of BD pathogenesis (Fig. 1A). At the tree root level, the diverse genetic variants in a subpopulation of the patients might functionally converge on a handful of key genes or pathways, which represent the trunk of the tree. At the tree crown level, abnormalities in one key gene and other genes it influences might cause different changes among brain regions, neural circuits or neuronal subtypes, which, together, cooperate to cause multi-layer pathophysiological changes that are representative of BD. The authors therefore think that identifying such key factors could be crucial to understanding the pathogenesis of BD at the genetic and neural-circuit levels.
To narrow down the candidate gene list, the authors performed a comparative analysis between their patient RNA-seq results and the reported genes involved in BD comorbidities (Fig. 1B). One important feature of BD is that ~40% patients suffer comorbid insulin metabolic syndrome, although the mechanisms have remained unknown. The authors hypothesized that the deficits of the key gene or signaling pathway in the brain could induce behavioral symptoms when they occurred in the brain, while generating symptoms of metabolic disorders when they occurred elsewhere in the body. This might be one reason why the two diseases co-occurred.
The authors searched public gene databases for genes that contribute to metabolic syndrome and investigated their expression in the patient iPSC-derived neurons, eventually focusing on 11 candidate genes (Fig. 1C). Among these genes, they found that Synaptotagmin-7 (Syt7) might be a candidate risk factor for behavioral abnormalities.
The authors then systematically investigated the behavioral defects of mice lacking Syt7 as well quantifiying levels of Syt7 both in neurons derived from induced pluripotent stem cells of 12 BD patients and the plasma of 20 patients. They found that the mice lacking Syt7 showed manic-like behavioral abnormalities in the dark and depressive-like behavioral abnormalities in the light, which is considered analogous to the mood cycles in bipolar disorder. Furthermore, the behavioral abnormalities shown by these mice could be efficiently treated by mood stabilizing drugs, namely olanzapine and lithium. The researchers also found the Syt7 was attenuated in the plasma samples of 20 patients and iPSC-derived neurons of 12 patients. Together, these results suggest that Syt7 likely plays a key role in bipolar-like behavioral abnormalities.
At present, the authors have been working to investigate the molecular mechanism employed by Syt7 and its downstream factors to induce manic and depressive behaviors in mice and patients, and their long-term goal is to make clear the mechanism of BD pathogenesis at the genetic and neural-circuit levels.
Dr. Jun Yao from the State Key Laboratory of Membrane Biology, School of Life Sciences; Tsinghua-Peking Joint Center for Life Sciences, McGovern Institute for Brain Research, Tsinghua University; and Dr. Fred Gage from the Salk Institute for Biological studies, are co-corresponding authors of the paper. Dr. Wei Shen, Dr. Qiu-Wen Wang, and Yao-Nan Liu, are co-first authors. All other co-authors made significant contributions to the paper.
Link to the paper: https://doi.org/10.1073/pnas.1918165117
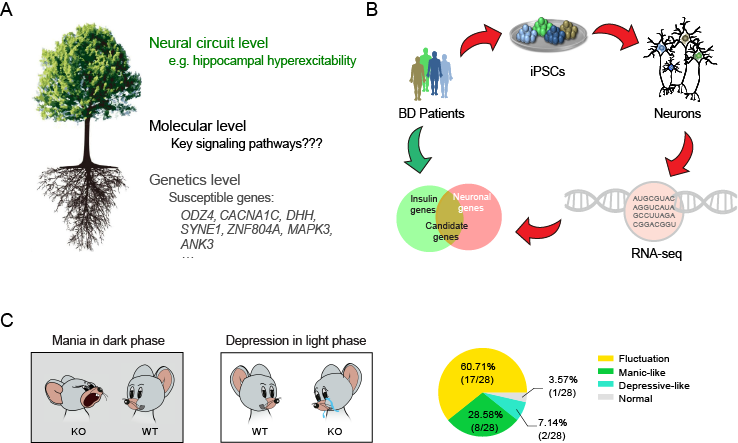
Figure 1. The tree model describing BD pathogenesis and the comorbidity-based screening for BD-related candidate genes
?
