On April 23th, 2018, a research team led by Dr. Wanli Liu from school of Life sciences, Tsinghua University published a research article entitled “PI(4,5)P2 determines the threshold of mechanical force-induced B cell activation” in Journal of Cell Biology. In this study, the group reported the molecular mechanism of low threshold of IgG-BCR activation in memory B cells by mechanical force. Zhengpeng Wan, a PhD student from PTN program (Peking University, Tsinghua University and the National Institute of Biological Sciences joint training program) is the first author, and Dr. Wanli Liu is the corresponding author of this article. This work integrated molecular biology, cell biology, high-resolution live-cell imaging and biophysics as well as other disciplines of cross-advantages by collaborating with domestic and international scientific groups.
B lymphocyte, as an important participant in the process of antibody immune response maintaining human health, is activated by pathogens through the membrane bound surface receptor called B-cell receptor (BCR). The previous work of this group revealed that B cells use BCRs to identify the physical and chemical properties of antigens. The activation of IgM-BCR expressed in na?ve B cells is highly dependent on mechanical forces with multi-threshold activation features. In contrast, the activation of isotype-switched IgG-BCR in memory B cells only requires a lower threshold. However, molecular mechanisms governing these differences remain to be identified.
In this research, the molecular mechanism IgG-BCR activation by lower mechanical force was studied systematically by combining the double-stranded DNA (dsDNA) based tension gauge tether (TGT) as a mechanical force sensor, the high speed high resolution imaging system based on total internal reflection, confocal fluorescence microscopy and super-resolution imaging technologies. The paper reported that the low threshold of IgG-BCR activation by mechanical force is highly dependent on tethering of the cytoplasmic tail of the IgG-BCR heavy chain (IgG-tail) to the plasma membrane (PM). Mechanistically, we show that the positively-charged residues in the IgG-tail play a crucial role by enriching phosphatidylinositol 4, 5-biphosphate (PIP2) into the membrane microdomains of IgG-BCRs. Indeed, manipulating the amounts of PIP2 within IgG-BCR membrane microdomains significantly altered the threshold and sensitivity of IgG-BCR activation. These studies reveals a lipid-dependent mechanism for determining the threshold of IgG-BCR activation by mechanical force. Additionally, these studies provide new insights into immune recognition, activation and regulation for B lymphocytes, helping people understand autoimmune diseases and providing important information on the pathogenesis of related diseases, which can lead to a better vaccine development based on this theoretical knowledge.
Dr. Wanli Liu’s group has been committed to the use of combining immunological and biochemical experimental approaches with cutting-edge high-resolution high-speed live cell and molecule imaging techniques as well as biophysical methods to investigate the function of lymphocytes. Following the publications in the Journal of Immunology (2013), European Journal of Immunology (2015), and eLife (2015, 2017) about immunological activation of B lymphocytes by mechanical force, this achievement is his new contribution to this field. The study is funded by the National Science Foundation of China, the Ministry of Science and Technology of People's Republic of China.
Link of the paper: http://jcb.rupress.org/content/early/2018/04/20/jcb.201711055

Figure. Hand drawing of PIP2 enrichment within IgG-BCR membrane microdomains lowers the mechanical force threshold of B cell activation. (Figure credit: Xingwang Zhao, Tsinghua University)
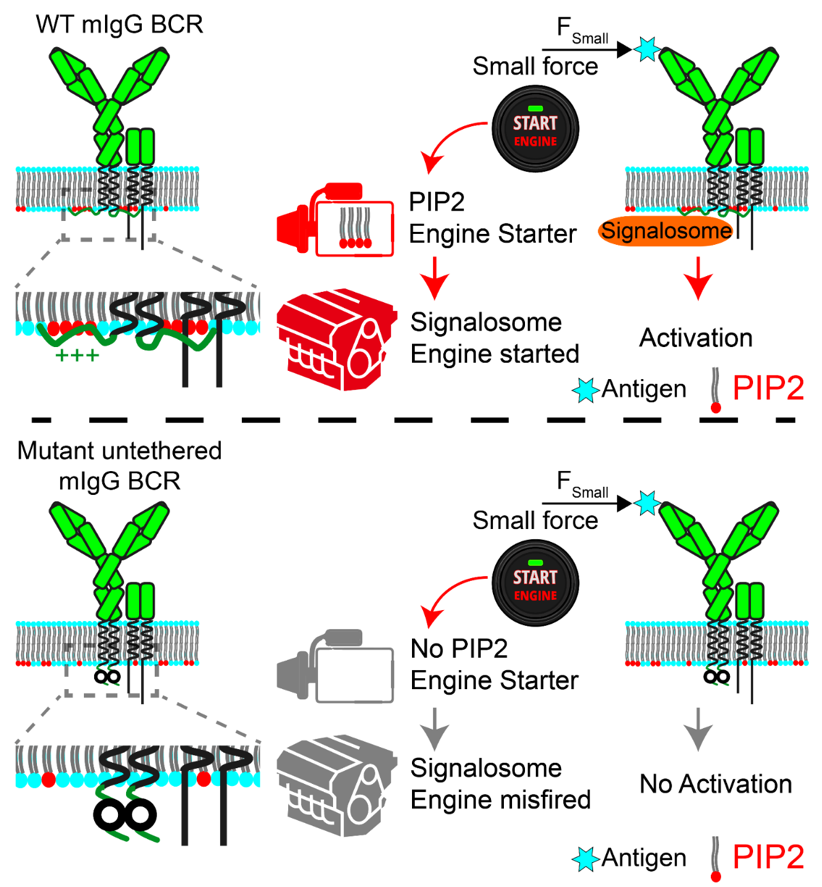
Figure. PIP2 enrichment regulates the mechanical force-induced B cell activation.
