Diffuse intrinsic pontine glioma (DIPG) is the most aggressive pediatric brainstem tumor with a median survival of less than 12 months; seeking effective therapy is still the focus of the field. Nearly 80% DIPG cells harbor H3K27M mutations, which caused global reduction in H3K27me3 levels, and H3K27M are considered as driver mutations for DIPG. This understanding has recently inspired substantial efforts to develop drugs for treating DIPG based on global modulation of the histone methylation/acetylation balance. Whilst promising, the findings from such studies have had limited translational impacts. Hence, screening drugs for treating DIPG is highly demanding.
In addition to the characteristic H3K27M mutations, up to 90% of H3.3K27M mutant DIPG harbor alterations in the p53 pathway, including mutations in TP53 (accounting for 65~69%) itself, as well as protein phosphatase, Mg2+/Mn2+-dependent 1D (PPM1D, accounting for 20%~28%). PPM1D truncating mutations promoted tumor progression in DIPG by destabilizing and inactivating p53. These evidences suggest that reactivating p53 tumor suppressive function in H3.3K27M DIPG cells might be a solution for DIPG therapy.

On February 15, 2024, Qiaoran Xi’s research group from Tsinghua University and Liwei Zhang’s research group from Beijing Tiantan Hospital, Capital Medical University published an article in Cancer Research entitled “Fimepinostat impairs NFκB and PI3K/AKT signaling and enhances gemcitabine efficacy in H3.3K27M-diffuse intrinsic pontine glioma”.
In this study, they used treatment-naïve patient-derived DIPG cell line provided by Tiantan hospital, and performed epigenetic compounds screening to identify that gemcitabine as a candidate. As a pyrimidine analog, gemcitabine stabilized and activated p53, including increasing chromatin accessibility for p53 at apoptosis-related loci. Notably, the H3.3K27M mutation facilitates gemcitabine-induced apoptosis in DIPG. However, gemcitabine induced the transcription of ERV (endogenous retrovirus), which might activate cGAS-STING signaling, and stimulated non-canonical NFκB signaling. A drug screen in gemcitabine-treated DIPG cells revealed that fimepinostat, a dual inhibitor of HDAC and PI3K, effectively suppressed the gemcitabine-induced NFκB signaling in addition to blocking PI3K/AKT activation. Combination therapy comprising gemcitabine and fimepinostat elicited synergistic anti-tumor effects in vitro and in orthotopic H3.3K27M DIPG xenograft models.
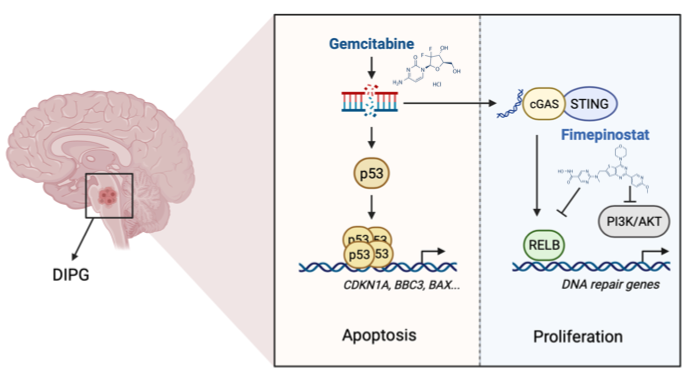
Fig: Activation p53 tumor suppressive function by gemcitabine and suppression of non-canonical NF-κB signaling using fimepinostat are potent therapeutic strategy for H3.3K27M DIPG.
Associate professor Qiaoran Xi from the School of Life Sciences, Tsinghua University and professor Liwei Zhang from Beijing Tiantan Hospital, Capital Medical University are the co-corresponding authors of this article. Dr. Dan Wang from Qiaoran Xi Lab is the first authors. Dr. Kun Yan, Ph.D. student Hongxing Yu, Haocheng Li, Wei Zhou and Shuning Guo from Qiaoran Xi Lab, postdoctoral Yaqiang Hong from Prof. 's Nian Liu research group, Yi Wang, Cheng Xu, Changcun Pan from Prof. 's Liwei Zhang research group, professor Yujie Tang from Shanghai Jiao Tong University School and professor Wei Wu provided an important contribution to the study. This work was supported by NSFC grant, MSTC grant, CLS program and Beijing Municipal Natural Science Foundation.
Link: https://doi.org/10.1158/0008-5472.CAN-23-0394
