TGF-β family proteins including Nodal are known as central regulators of early development in metazoans. For example, Nodal signaling initiates gastrulation in vivo and promotes embryonic stem cells (ESCs) to differentiate towards mesendoderm fates in vitro; these regulatory processes are mediated by the transcriptional upregulation of so-called “mesendoderm lineage determining transcription factors” (LDTFs) . Yet our understanding of the scope of Nodal signaling’s downstream targets and associated physiological mechanisms in specifying developmentally appropriate cell fates is far from complete.
With the innovation and development of high-throughput transcriptome sequencing (RNA-seq) and bioinformatics technology in recent years, a large number of long noncoding RNAs (LncRNAs) have been identified. At the same time, some studies have shown that some non-coding genes can be translated into functional micropeptides and play important functions. However, whether there are some LncRNAs or micropeptides regulated by the Nodal signaling pathway play a role in the self-renewal and differentiation of embryonic stem cells still needs to be further elucidated.
On July 9, 2022, Qiaoran Xi’s research group in Tsinghua University published a research article in Nature Communications entitled “A Nodal enhanced micropeptide NEMEP regulates glucose uptake during mesendoderm differentiation of embryonic stem cells” In this study, they identified a highly conserved, transmembrane micropeptide—NEMEP—as a direct target of Nodal signaling in mesendoderm differentiation of mouse embryonic stem cells (mESCs), and this micropeptide is essential for mesendoderm differentiation. They showed that NEMEP interacts with the glucose transporters GLUT1/GLUT3 and promotes glucose uptake likely through these interactions. Thus, beyond expanding the scope of known Nodal signaling targets in early development and showing that this target micropeptide augments the glucose uptake during mesendoderm differentiation, their study provides a clear example for the direct functional impact of altered glucose metabolism on cell fate determination.
Associate professor Qiaoran Xi from the School of Life Sciences, Tsinghua University is the corresponding author of this article. Dr. Haipeng Fu is the first author. Tingyua Wang, Dr. Xiaohui Kong, Dr. Kun Yan from Qiaoran Xi Lab, Dr. Yafei Yuan from Beijing Advanced Innovation Center for Structural Biology in Tsinghua University, Dr. Nan Wang from Prof. Kehkooi Kee Lab from School of medicine in Tsinghua University, Dr. Yang Yang and Dr Jingyi Cao from Prof. Zhi John Lu Lab from School of Life Sciences in Tsinghua University provide an important contribution to the study. This work was supported by NSFC grant and CLS program.
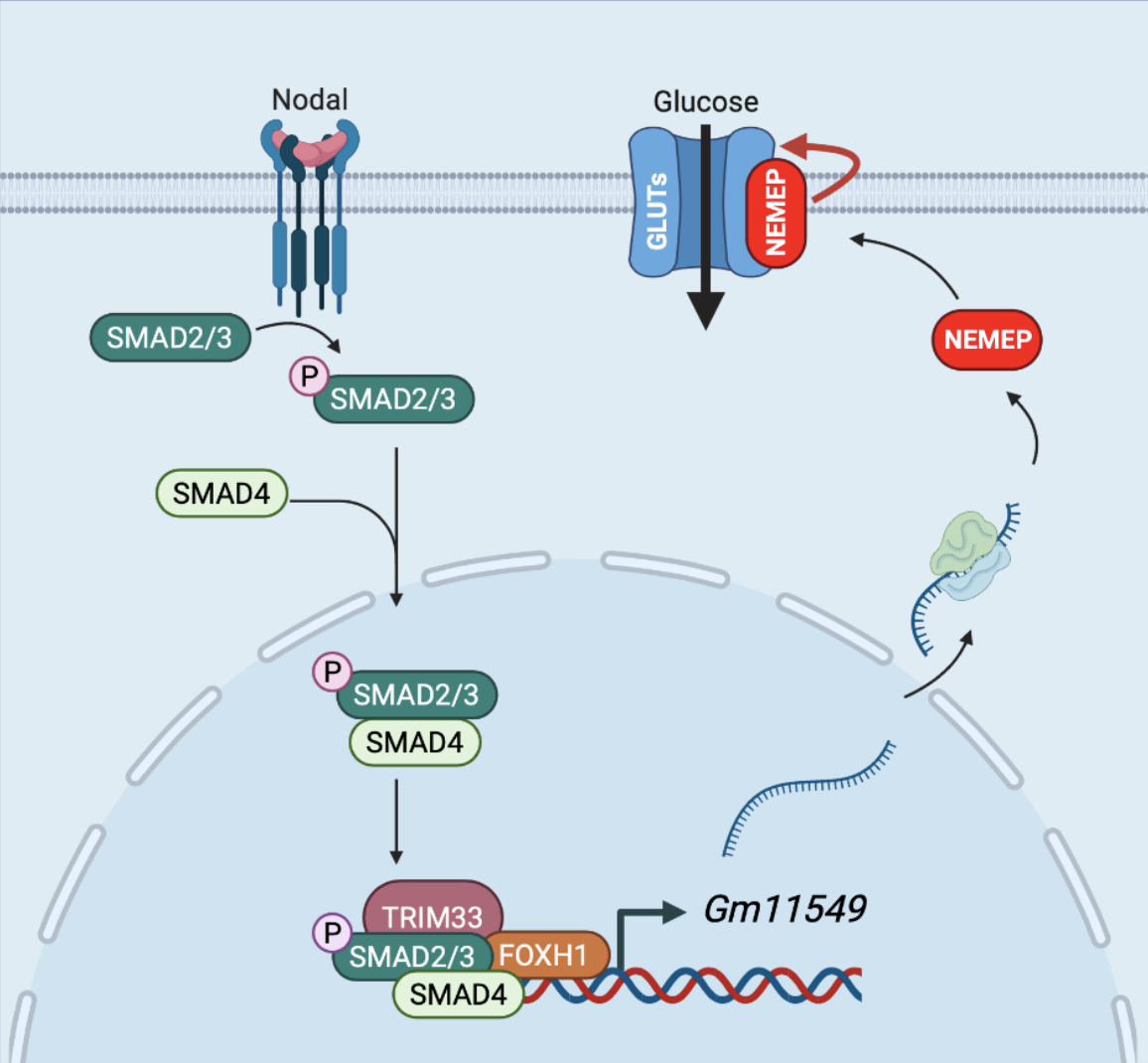
Working model for the role of NEMEP during mesendoderm differentiation (Created with BioRender.com). A highly conserved putative lncRNA Gm11549 is specifically induced by Nodal signaling during mesendoderm differentiation and it is essential for mesendoderm differentiation in mESCs. There is a “ORF” hidden in Gm11549 that codes a transmembrane micropeptide, we named NEMEP. NEMEP functions by interacting with GLUT1 and GLUT3, and augment the glucose uptake capacity of glucose transporter proteins to meet the energy needs during mesendoderm differentiation.
Link: https://doi.org/10.1038/s41467-022-31762-x
or PDF:https://www.nature.com/articles/s41467-022-31762-x.pdf.
