Comprehensive annotations of transcriptomes have revealed a vast number of long non-coding RNAs (lncRNAs) in various species. For the past years, extensive studies have uncovered hundreds of lncRNAs in human cells with various molecular and cellular functions. Many of these lncRNAs have been emerging as fine-tuners of key pathways related to a variety of processes, such as development and complex diseases such as cancer. Among them, physiologically relevant lncRNAs with fundamental and indispensable functions are still rare.
Recently, Dr. Xuerui Yang’s group in the School of Life Sciences at Tsinghua University has uncovered the indispensable role of a previously uncharacterized lncRNA, renamed as LETN, in shaping the organized nucleolar structure and sustaining the nucleolar functions required for cell proliferation. The underlying machinery based on a mutual dependency between the lncRNA LETN and a highly critical nucleolar protein NPM1 was published online by Cell Research on January 11, 2020, with the title Mutual dependency between lncRNA LETN and protein NPM1 in controlling the nucleolar structure and functions sustaining cell proliferation. Link to the article: https://www.nature.com/articles/s41422-020-00458-6
This work was originated from a comprehensive survey of the lncRNA functions in various cancers, performed Dr. Xuerui Yang’s dry lab with the multi-omics cancer cohort data from the large consortiums such as TCGA. Given the vast number and extremely limited prior knowledge of the noncoding transcriptome, such a hypothesis-free strategy, if executed properly, would be of high value for honing in on specific lncRNAs and guiding the mechanistic studies. Indeed, previously their efforts have generated valuable insights that led to the discovery of HNRNPC in controlling the Alu RNA-originated immune-stimulatory dsRNA in breast cancer cells (The EMBO Journal, 2018) and the lncRNA NEAT in promoting cell cycle via AGRN in prostate cancer cells (Cancer Research, 2018). Previously, the group have also used the same methodology to infer the DNA methylation sites that modulate the transcriptional regulatory circuits (Cell Reports, 2019). Lately, the similar pipeline again revealed a quite surprising prediction of the lncRNA RP11-196G18.22, which was renamed as LETN later, being the most potent and a global modulator of the gene expression program in liver cancer.
Inspired and guided by the prediction above, the wet-lab part of Xuerui Yang group then performed extensive investigations on the lncRNA LETN. The study led to a series of striking discoveries illustrating a highly critical position of the lncRNA in the fundamental processes in the nucleolus. Specifically, being upregulated in multiple types of cancer, LETN resides in the nucleolus via direct binding with NPM1. LETN plays a critical role in facilitating the formation of NPM1 pentamers, which are essential building blocks of the nucleolar granular component and control the nucleolar functions. Fundamental processes such as ribosomal RNA synthesis and chromatin remodeling take place in the nucleolus, which is hyperactive in fast-proliferating cells. Together with the team members, Xianteng Wang in Xuerui Yang group, who is the first author of the paper, found that repression of LETN or NPM1 led to identical and strong shift of the nucleolar morphology and arrest of the nucleolar functions, which led to proliferation inhibition of human cancer cells and neural progenitor cells. In brief, the study uncovered the mutual functional dependency between the key multi-functional protein NPM1 and a previously uncharacterized lncRNA.
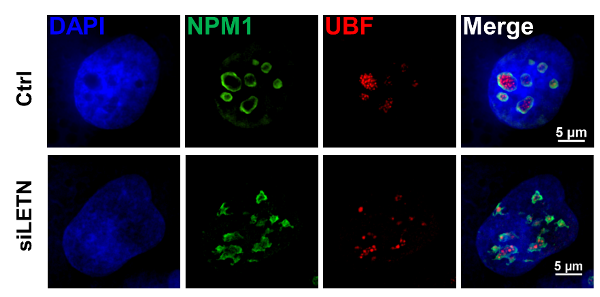
Knock-down of LETN largely disrupted the nucleolar NPM1 distribution patterns
Interestingly, comparative genomic analyses further showed that this inter-dependency between LETN and NPM1 is associated with the evolutionarily new variations of NPM1, which are coincidental with the emergence of LETN in higher primates. Such apparent coevolution between the non-coding genome and the protein renders an additional yet critical layer of regulation in human and potentially other higher primates, for the nucleolar functions that sustain fast cell proliferation in both cancerous and normal developmental processes that require hyperactive nucleoli.
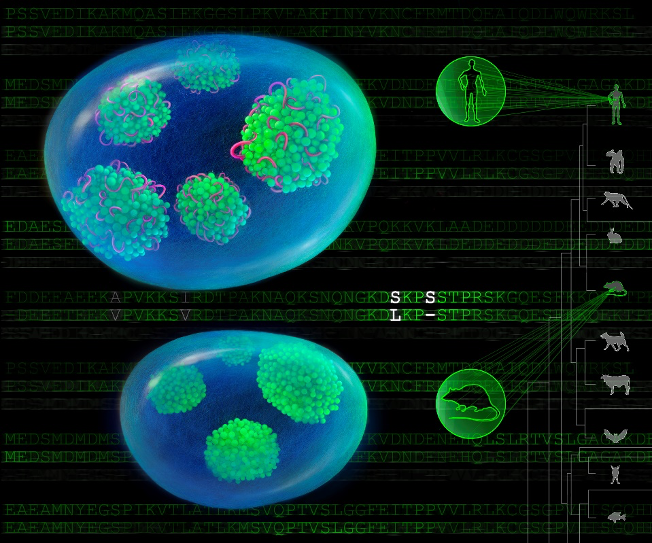
A small yet pivotal variation in human nucleolar protein NPM1 renders its full dependency on the human-specific non-coding RNA LETN
Xianteng Wang, a PhD student in Xuerui Yang’s lab, is the first authors of the paper. Other coauthors, including Xiaoling Hu, Wanlu Song, Hui Xu, and Zhengtao Xiao, contributed significantly to the data analysis part of this work. Other collaborators include Qin Shen’s group at Tongji University and Haitao Zhao in the Department of Liver Surgery at Peking Union Medical College Hospital. The study was financially supported by the national key research and development program, Precision Medicine Project, the National Natural Science Foundation of China, the Tsinghua University Initiative Scientific Research Program. The study also received supports from Tsinghua University Branch of China National Center for Protein Sciences (Beijing) and Tsinghua University Technology Center for Protein Research, including the core facilities of Genome Sequencing and Analysis, Biocomputing, Cell Imaging, Protein Preparation and Identification, Cell Function Analyzing, and Protein Chemistry and Proteomics, and the Lab Animal Center at Tsinghua University.
